Abstract
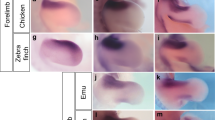
Morphological characters are the result of developmental gene expression. The identity of a character is ultimately grounded in the gene regulatory network directing development and thus whole-genome gene expression data can provide evidence about character identity. This approach has been successfully used to assess cell-type identity1,2,3. Here we use transcriptomic data to address a long-standing uncertainty in evolutionary biology, the identity of avian wing digits4,5. Embryological evidence clearly identifies the three wing digits as developing from digit positions 2, 3 and 4 (ref. 6), whereas palaeontological data suggest that they are digits I, II and III7. We compare the transcriptomes of the wing and foot digits and find a strong signal that unites the first wing digit with the first foot digit, even though the first wing digit develops from embryological position 2. Interestingly, our transcriptomic data of the posterior digits show a higher degree of differentiation among forelimb digits compared with hindlimb digits. These data show that in the stem lineage of birds the first digit underwent a translocation from digit position 1 to position 2, and further indicate that the posterior wing digits have unique identities contrary to any model of avian digit identity proposed so far5,8.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Novershtern, N. et al. Densely interconnected transcriptional circuits control cell states in human hematopoiesis. Cell 144, 296–309 (2011)
Cherbas, L. et al. The transcriptional diversity of 25 Drosophila cell lines. Genome Res. 21, 301–314 (2011)
Alizadeh, A. A. et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403, 503–511 (2000)
Wagner, G. P. The developmental evolution of avian digit homology: an update. Theory Biosci. 124, 165–183 (2005)
Young, R. L., Bever, G. S., Wang, Z. & Wagner, G. P. Identity of the avian wing digits: problems resolved and unsolved. Dev. Dyn. 240, 1042–1053 (2011)
Burke, A. C. & Feduccia, A. Developmental patterns and the identification of homologies in the avian hand. Science 278, 666–668 (1997)
Wagner, G. P. & Gauthier, J. A. 1,2,3 = 2,3,4: a solution to the problem of the homology of the digits in the avian hand. Proc. Natl Acad. Sci. USA 96, 5111–5116 (1999)
Tamura, K. et al. Embryological evidence identifies wing digits in birds as digits 1, 2, and 3. Science 331, 753–757 (2011)
Burke, A. C., Nelson, C. E., Morgan, B. A. & Tabin, C. Hox genes and the evolution of vertebrate axial morphology. Development 121, 333–346 (1995)
Mansfield, J. H. & Abzhanov, A. Hox expression in the American alligator and evolution of archosaurian axial patterning. J. Exp. Zool. B 314, 629–644 (2010)
Averof, M. & Akam, M. Hox genes and the diversification of insect and crustacean body plans. Nature 376, 420–423 (1995)
Arendt, D. The evolution of cell types in animals: emerging principles from molecular studies. Nature Rev. Genet. 9, 868–882 (2008)
Sugino, K. et al. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nature Neurosci. 9, 99–107 (2006)
Palmer, C., Diehn, M., Alizadeh, A. A. & Brown, P. O. Cell-type specific gene expression profiles of leukocytes in human peripheral blood. BMC Genomics 7, 115 (2006)
Wagner, G. P. The developmental genetics of homology. Nature Rev. Genet. 8, 473–479 (2007)
Young, R. L. & Wagner, G. P. Why ontogenetic homology criteria can be misleading: lessons from digit identity transformations. J. Exp. Zool. B 316B, 165–170 (2011)
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010)
Welten, M. C., Verbeek, F. J., Meijer, A. H. & Richardson, M. K. Gene expression and digit homology in the chicken embryo wing. Evol. Dev. 7, 18–28 (2005)
Tickle, C. Making digit patterns in the vertebrate limb. Nature Rev. Mol. Cell Biol. 7, 45–53 (2006)
Vargas, A. O. & Fallon, J. F. Birds have dinosaur wings: The molecular evidence. J. Exp. Zool. B 304B, 86–90 (2005)
Vargas, A. O. et al. The evolution of HoxD-11 expression in the bird wing: insights from Alligator mississippiensis . PLoS ONE 3, e3325 (2008)
Uejima, A. et al. Anterior shift in gene expression precedes anteriormost digit formation in amniote limbs. Dev. Growth Differ. 52, 223–234 (2010)
Montavon, T., Le Garrec, J. F., Kerszberg, M. & Duboule, D. Modeling Hox gene regulation in digits: reverse collinearity and the molecular origin of thumbness. Genes Dev. 22, 346–359 (2008)
Firulli, B. A. et al. Altered Twist1 and Hand2 dimerization is associated with Saethre-Chotzen syndrome and limb abnormalities. Nature Genet. 37, 373–381 (2005)
Zhu, L., Zhou, G., Poole, S. & Belmont, J. W. Characterization of the interactions of human ZIC3 mutants with GLI3. Hum. Mutat. 29, 99–105 (2008)
Tzchori, I. et al. LIM homeobox transcription factors integrate signaling events that control three-dimensional limb patterning and growth. Development 136, 1375–1385 (2009)
Suzuki, R. & Shimodaira, H. Pvclust: an R package for assessing the uncertainty in hierarchical clustering. Bioinformatics 22, 1540–1542 (2006)
Wang, L. K. et al. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26, 136–138 (2010)
Hargrave, M., Bowles, J. & Koopman, P. In situ hybridization of whole-mount embryos. Methods Mol. Biol. 326, 103–113 (2006)
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010)
Dahn, R. D. & Fallon, J. F. Interdigital regulation of digit identity and homeotic transformation by modulated BMP signaling. Science 289, 438–441 (2000)
Suzuki, T., Hasso, S. M. & Fallon, J. F. Unique SMAD1/5/8 activity at the phalanx-forming region determines digit identity. Proc. Natl Acad. Sci. USA 105, 4185–4190 (2008)
Hamburger, V. & Hamilton, H. L. A series of normal stages in the development of the chick embryo. J. Morphol. 88, 49–92 (1951)
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995)
Young, M. D., Wakefield, M. J., Smyth, G. K. & Oshlack, A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 11, R14 (2010)
McMahon, A. R. & Merzdorf, C. S. Expression of the zic1, zic2, zic3, and zic4 genes in early chick embryos. BMC Res. Notes 3, 167 (2010)
Abellán, A. et al. Olfactory and amygdalar structures of the chicken ventral pallium based on the combinatorial expression patterns of LIM and other developmental regulatory genes. J. Comp. Neurol. 516, 166–186 (2009)
Nelson, C. E. et al. Analysis of Hox gene expression in the chick limb bud. Development 122, 1449–1466 (1996)
Acknowledgements
The authors thank J. Noonan for discussions on this project, K. Cooper for experimental assistance and N. Carriero for read-mapping assistance and C. Tabin for providing us with the Hoxd12 probe. The authors are also grateful for the technical support for this project by the Yale Center for Genomic Analysis. The financial support by the Yale Science Development Fund is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Z.W. performed the experiments and data analysis and participated in design of the study. R.L.Y., H.X. and G.P.W. participated in data analysis. G.P.W. conceived and designed the study and supervised the work. All authors discussed the results and made substantial contributions to the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-10 with legends, Supplementary Table 1, a Supplementary Discussion and Supplementary References. (PDF 989 kb)
Rights and permissions
About this article
Cite this article
Wang, Z., Young, R., Xue, H. et al. Transcriptomic analysis of avian digits reveals conserved and derived digit identities in birds. Nature 477, 583–586 (2011). https://doi.org/10.1038/nature10391
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature10391
This article is cited by
-
Gene expression changes during the evolution of the tetrapod limb
Biologia Futura (2022)
-
‘Dinosaur-bird’ macroevolution, locomotor modules and the origins of flight
Journal of Iberian Geology (2021)
-
Colymbosaurines from the Upper Jurassic of European Russia and their implication for palaeobiogeography of marine reptiles
Palaeobiodiversity and Palaeoenvironments (2020)
-
A single-cell transcriptomic atlas of the developing chicken limb
BMC Genomics (2019)
-
Evolution of the avian digital pattern
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.